
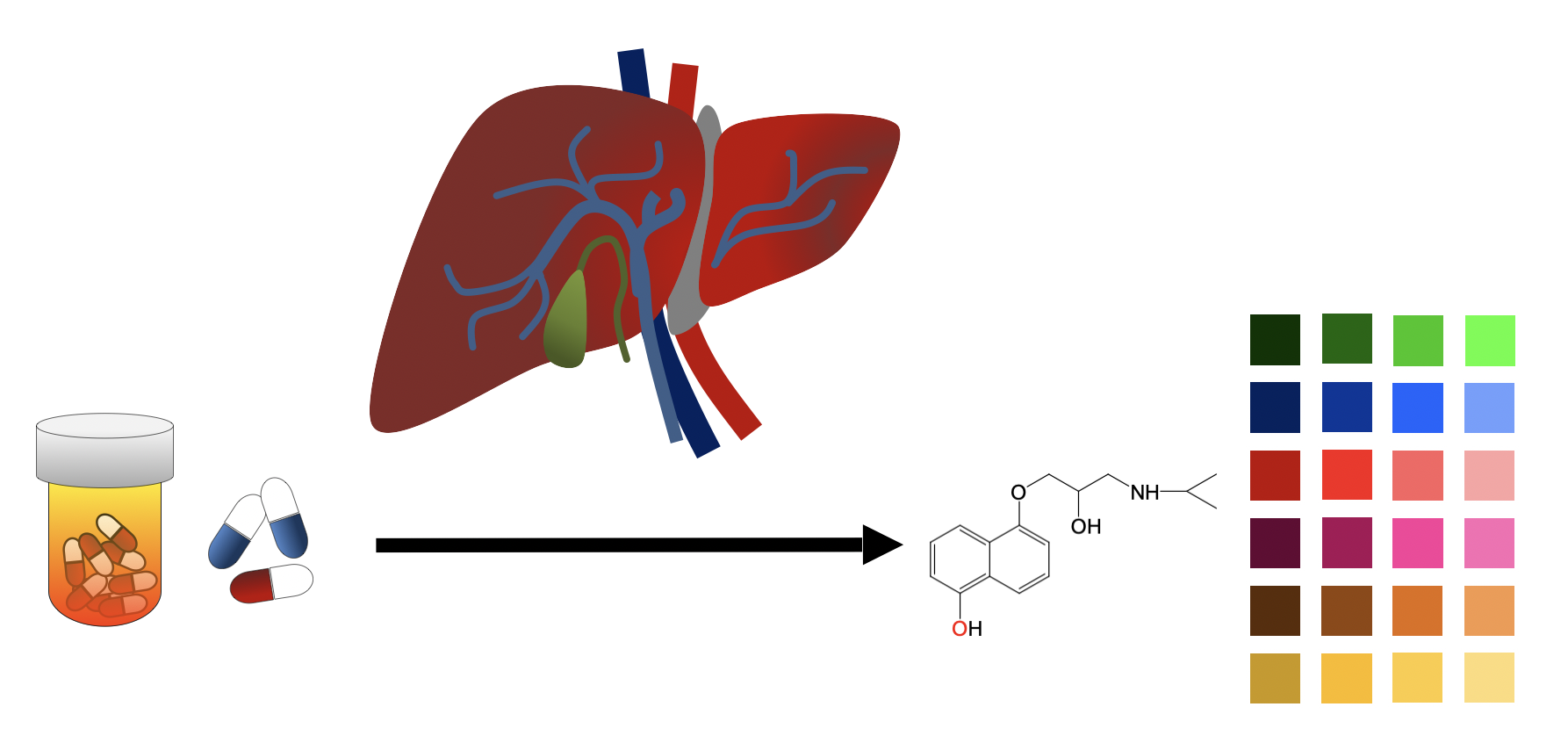
Fungal unspecific peroxygenases (UPOs)
Fewer requirements and numerous reactions
Peroxygenases are enzymes that perform complex C-H oxyfunctionalizations with a high degree of selectivity, simply triggered by hydrogen peroxide. The broad portfolio of reactions performed by UPOs, along with their fewer demanding requirements, place these enzymes in a unique position to boost a variety of particularly innovative chemical reactions.

Laccases
Higher redox potential and better performance

Laccases are ecological enzymes that oxidise a wide array of compounds using oxygen from the air and releasing water as a by-product. As a result, there are dozens of applications for these enzymes in the bioremediation, textile, pulp and paper, chemical and pharmaceutical industries.
Our evolved mutant laccases have a high-redox potential, which allows them to work with high potential redox mediators and they have been designed ad-hoc to improve their performance, including tolerance to high temperature and extreme pH.
Versatile peroxidases (VPs)
Several catalytic sites in a single enzyme
Our evolved VPs have high redox potentials and improved features, such as higher thermostabilities, resistance to extreme pHs and resistance to oxidative inactivation. These properties and their tremendous catalytic performance make them perfect for applications in bioremediation, food, organic synthesis, and biosensor development.

Aryl alcohol oxidases
Ideal for chiral mixtures separation

The activity of aryl alcohol oxidase (AAO) on a wide range of alcohols and aldehydes, together with the production of H2O2 from atmospheric O2, has awakened biotechnological interest with regards several potential industrial applications. The gradual release of H2O2 can be encompassed in self-sufficient systems for efficient cascade reactions.
The mutant evolved AAOs that we develop have high enantioselectivity regarding different primary and secondary aromatic substrates, allowing chiral mixtures of alcohols to be resolved.
Cutinases
Degradation of recalcitrant polymers
Cutinases are versatile enzymes that can catalyze hydrolysis reactions with a wide range of substrates, such as short-chain soluble esters, water-insoluble medium and long-chain triacylglycerols, and synthetic polyester polymers such as PET, polyethylene furan-2,5-dicarboxylate (PEF), polybutylene succinate (PBS), etc. Additionally, other factors such as high activity and thermostability made cutinases potential enzymes for the degradation of synthetic polymers.

 To learn more, see some of our publications:
To learn more, see some of our publications: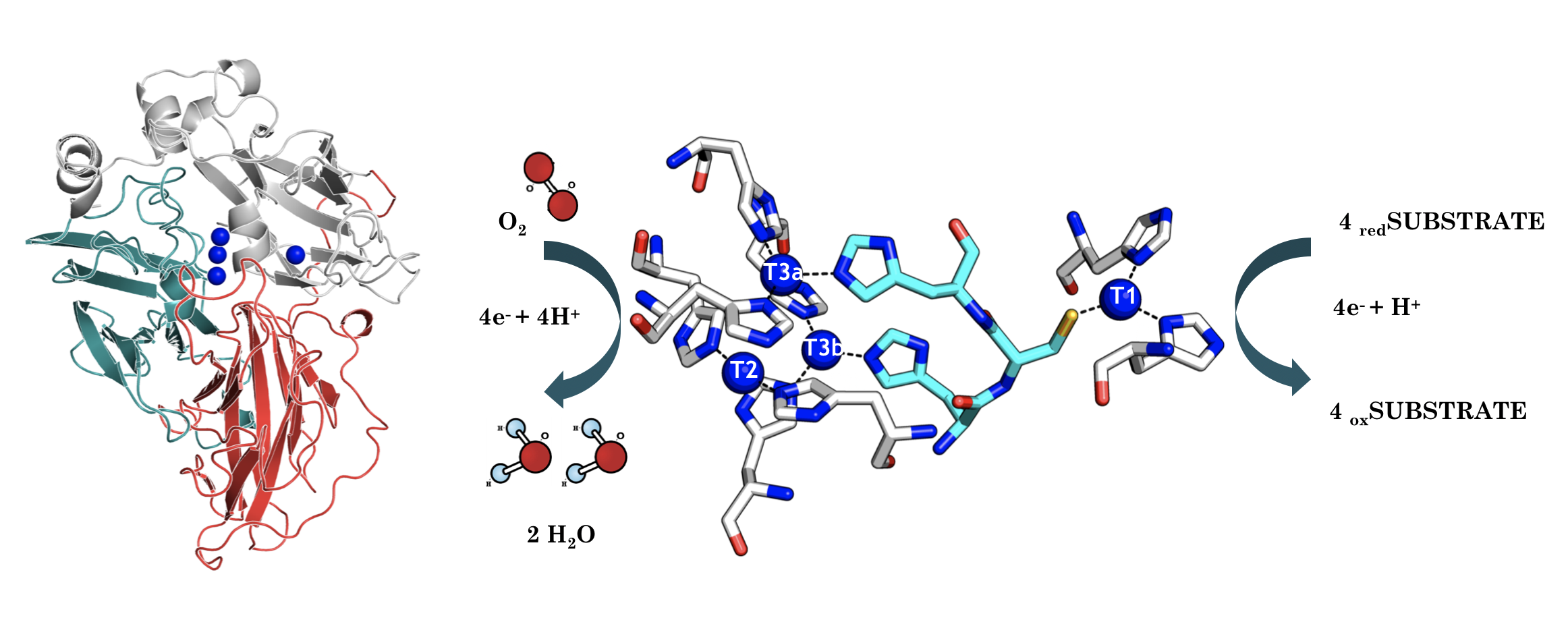 To learn more:
To learn more: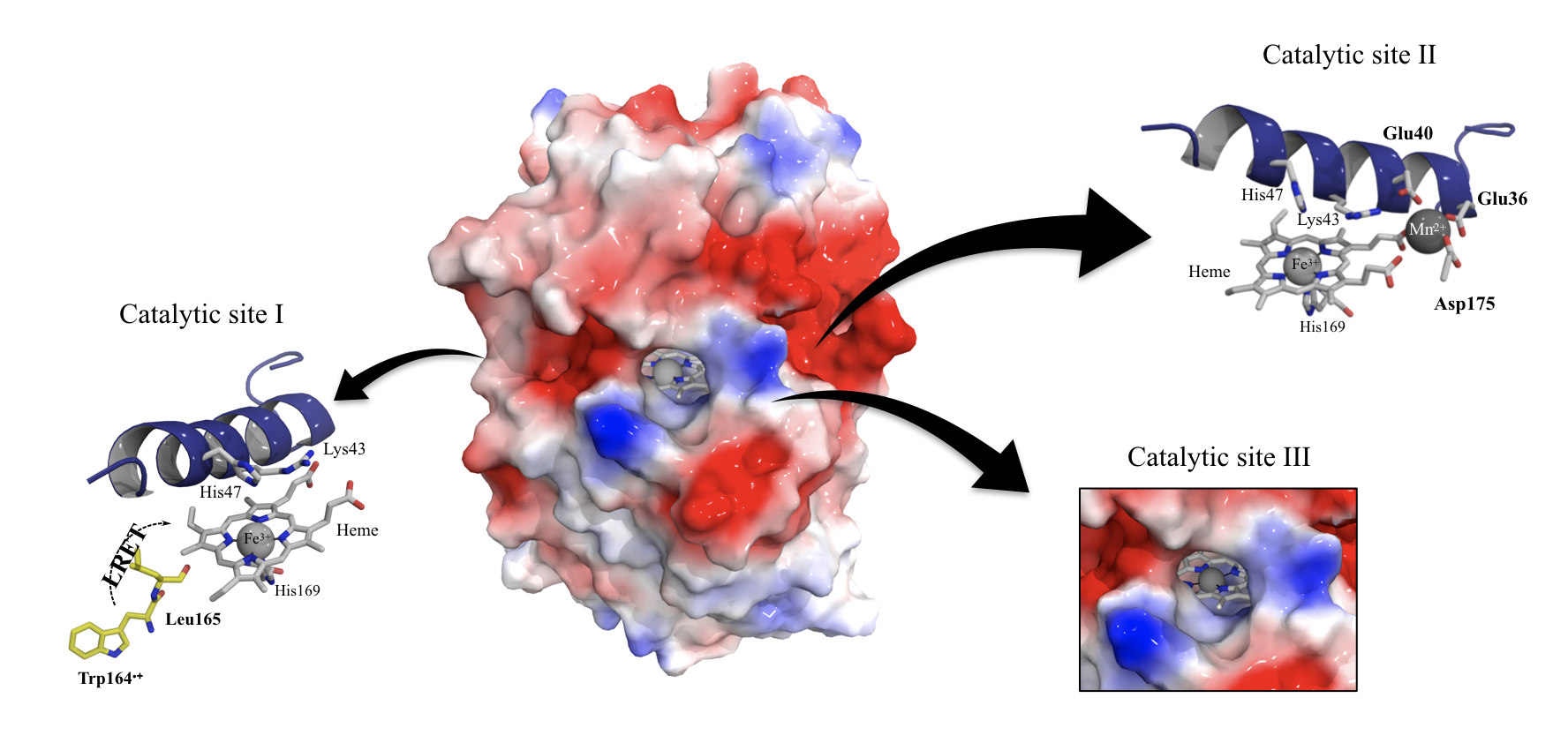 To learn more:
To learn more: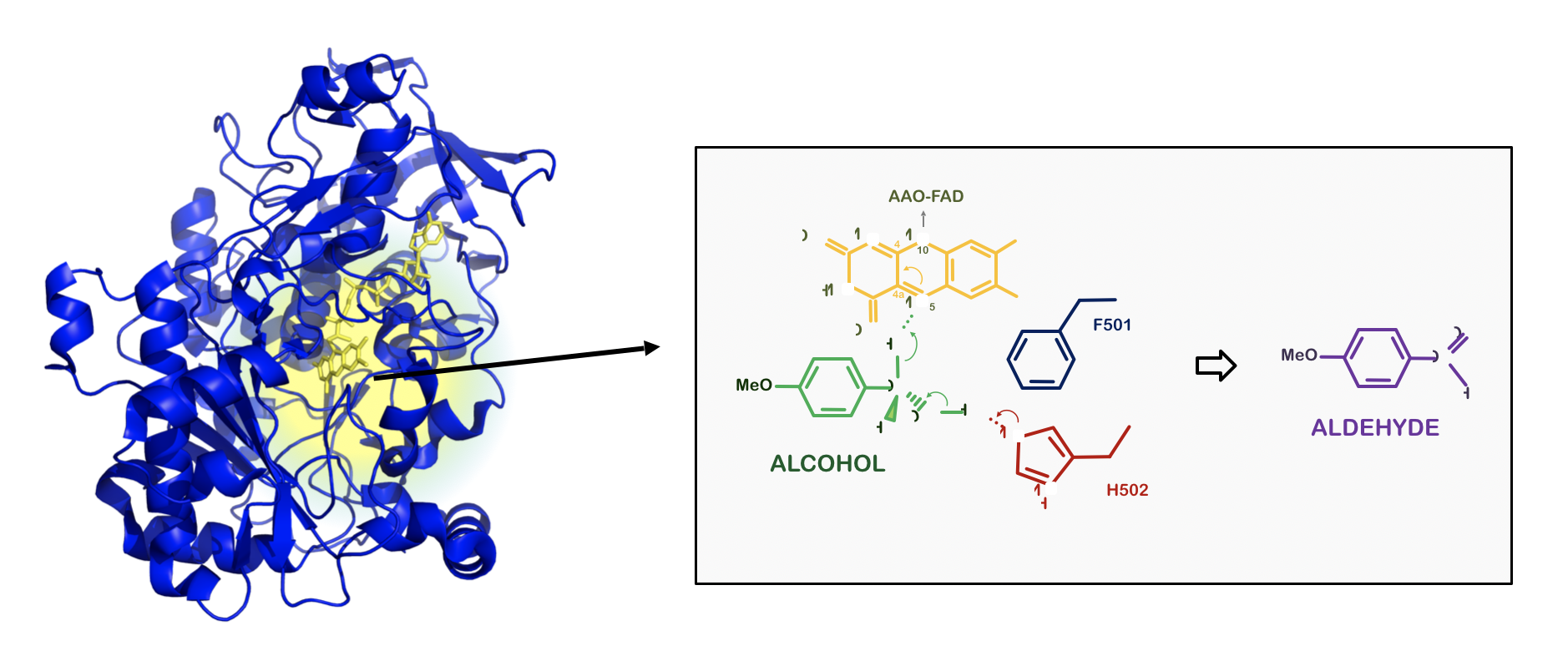 To learn more:
To learn more: