NEW

96 REACTIONS
Laccases are multi-copper containing oxidoreductases that oxidize a variety of different compounds, using only oxygen from the air and releasing water as the only by-product. Indeed, they are considered ideal green biocatalysts with broad applications in fields that include bioremediation, organic synthesis, biofuel cells or biosensors, textiles, pulp and paper, food and cosmetics (doi/10.1111/1751-7915.12422).
EVO LACkit is the first laccase screening kit on the market that contains an exclusive collection of laccases from white-rot fungi, all of which have a high-redox potential to oxidize a wide range of organic and inorganic molecules.
The 16 laccase variants used in the EVO LACkit are the product of extensive directed evolution studies aimed at engineering different facets of these enzymes, including their substrate promiscuity, catalytic activity or stability in response to pH, temperature and the presence of organic solvents.
Our variants are not only capable of performing classic laccase oxidation of phenols, polyphenols, aromatic amines and other electron-rich substrates but remarkably, they act efficiently in more challenging reactions with novel substrates or under reaction conditions in which other laccases fail. For bulky substrates (e.g. polymers) or compounds with high redox potentials, we strongly suggest enhancing the oxidative capacity of our laccase variants by using diffusible redox mediators and employing a laccase mediator system. To this end, three redox mediators that employ different oxidative mechanisms are also provided in the EVO LACkit for ease of use.

Laccase mediator system

The three mediators included in the EVOLac Kit and their distinct reaction mechanisms
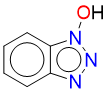
HBT – Hydrogen transfer mechanism

TEMPO – Ionic mechanism
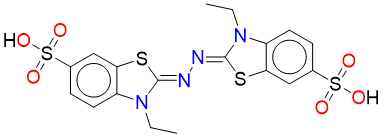
ABTS – Electron transfer mechanism
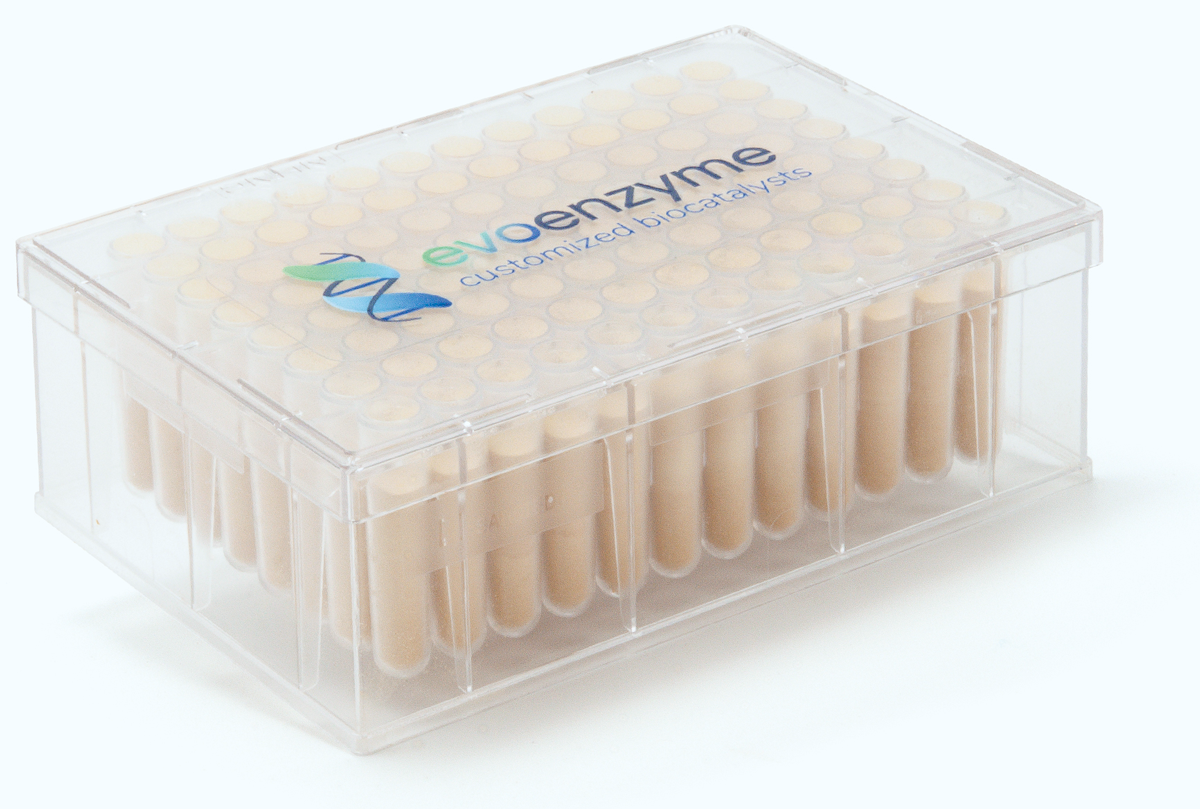
EVO LACkit is presented in 96-deep well plate format to make your reaction analysis rapid and cost-effective. All laccase variants in the EVO LACkit have been recombinantly produced in yeast (Saccharomyces cerevisiae). Should you find a positive hit, you can contact us so that we can work together to provide you with the mutant of your choice in larger amounts. Moreover, EvoEnzyme offers an additional service, designing your selected laccase a la carte to improve its performance in your given biotransformation.
Content of the EVO LACkit
- 16 unique and diverse high-redox potential laccases in lyophilized form organized in 96-deep well plate (6X each enzyme), allowing to screen six test compounds with each laccase variant.
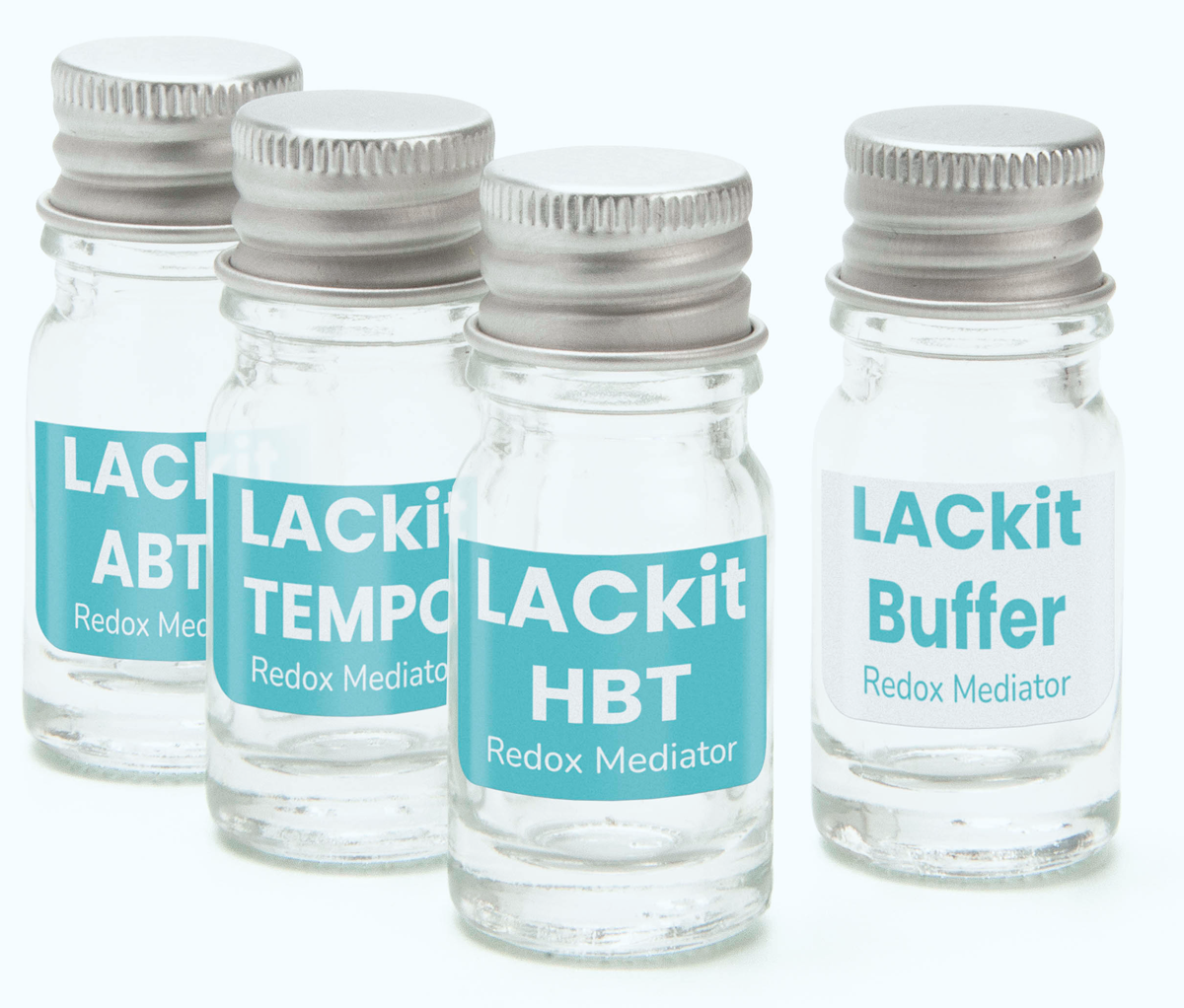
- Reaction buffer vial.
- Redox mediators: HBT, TEMPO, ABTS.
Some laccase engineering case studies
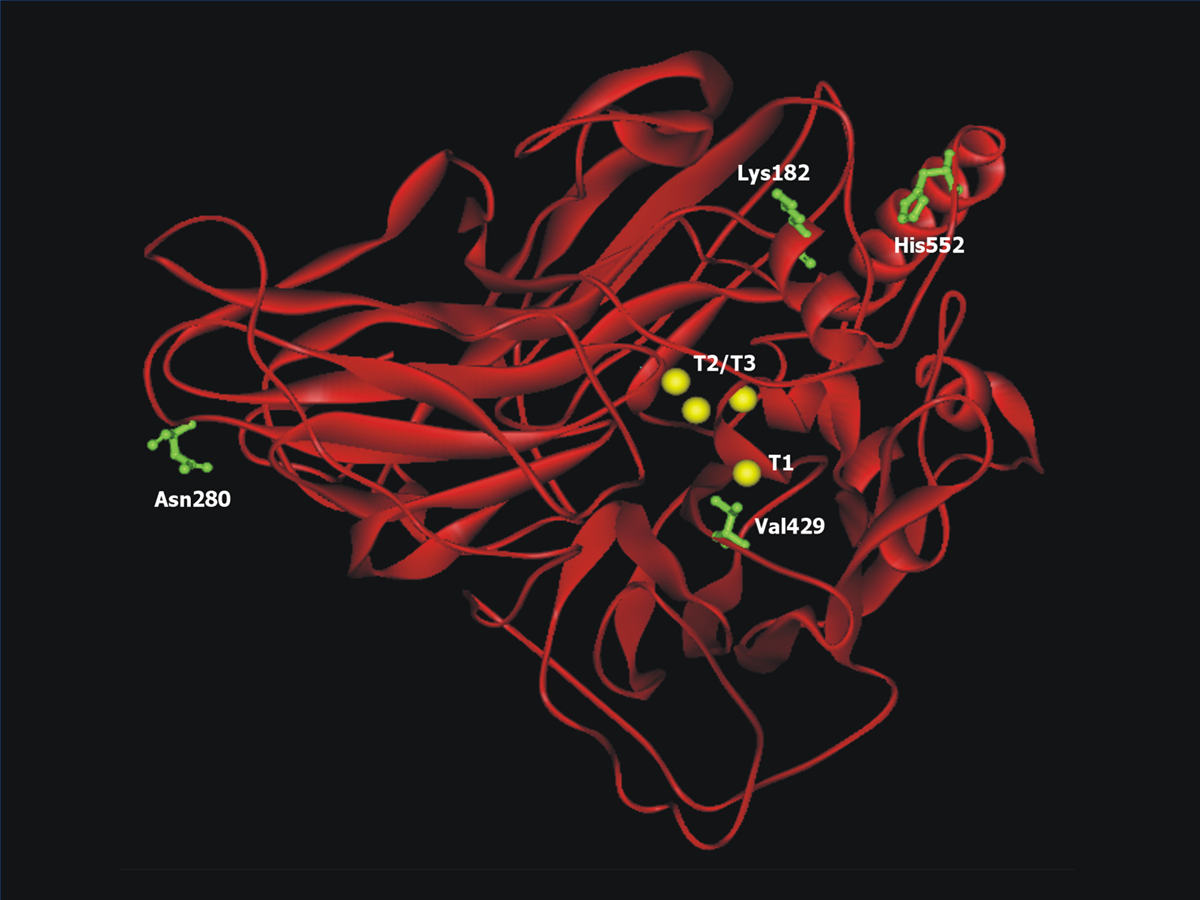
In vitro evolution of a fungal laccase in high concentrations of organic cosolvents
Zumárraga, M., Bulter, T., Shleev, S., Polaina, J., Martínez-Arias, A., Plou, F. J., Ballesteros, A., & Alcalde, M. (2007). In vitro evolution of a fungal laccase in high concentrations of organic cosolvents.
Chemistry & biology, 14(9), 1052–1064.
https://doi.org/10.1016/j.chembiol.2007.08.010.
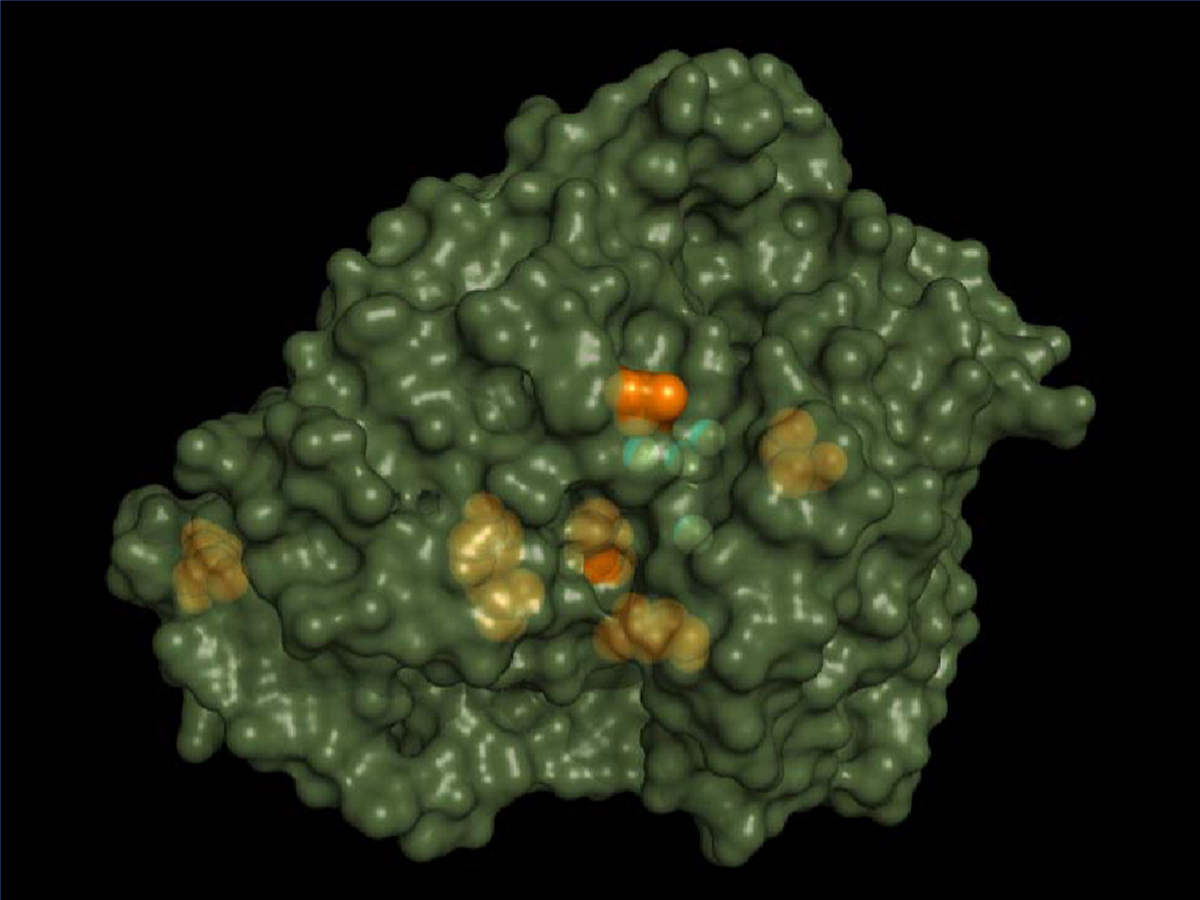
Laboratory evolution of high-redox potential laccases
Maté, D., García-Burgos, C., García-Ruiz, E., Ballesteros, A. O., Camarero, S., & Alcalde, M. (2010). Laboratory evolution of high-redox potential laccases.
Chemistry & biology, 17(9), 1030–1041.
https://doi.org/10.1016/j.chembiol.2010.07.010.
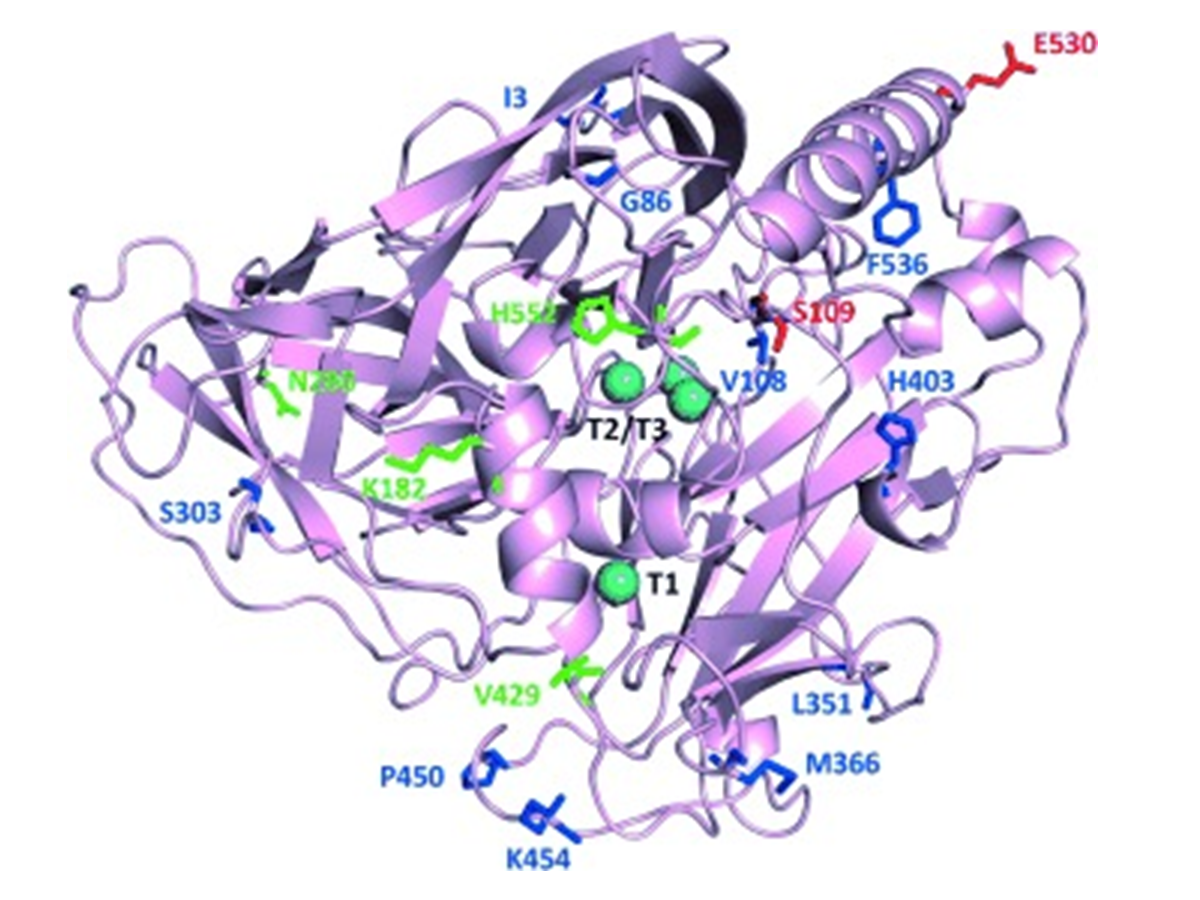
Widening the pH activity profile of a fungal laccase by directed evolution
Torres-Salas, P., Mate, D. M., Ghazi, I., Plou, F. J., Ballesteros, A. O., & Alcalde, M. (2013). Widening the pH activity profile of a fungal laccase by directed evolution.
Chembiochem : a European journal of chemical biology, 14(8), 934–937.
https://doi.org/10.1002/cbic.201300102.

Blood tolerant laccase by directed evolution
Mate, D. M., Gonzalez-Perez, D., Falk, M., Kittl, R., Pita, M., De Lacey, A. L., Ludwig, R., Shleev, S., & Alcalde, M. (2013). Blood tolerant laccase by directed evolution.
Chemistry & biology, 20(2), 223–231.
https://doi.org/10.1016/j.chembiol.2013.01.001.

Increasing Redox Potential, Redox Mediator Activity, and Stability in a Fungal Laccase by Computer-Guided Mutagenesis and Directed Evolution
Mateljak, I., Monza, E., Lucas, M. F., Guallar, V., Aleksejeva, O., Ludwig, R., Leech, D., Shleev, S., & Alcalde, M. (2019). Increasing Redox Potential, Redox Mediator Activity, and Stability in a Fungal Laccase by Computer-Guided Mutagenesis and Directed Evolution.
ACS Catalysis, 9(5), 4561-4572.
https://doi.org/10.1021/acscatal.9b00531.
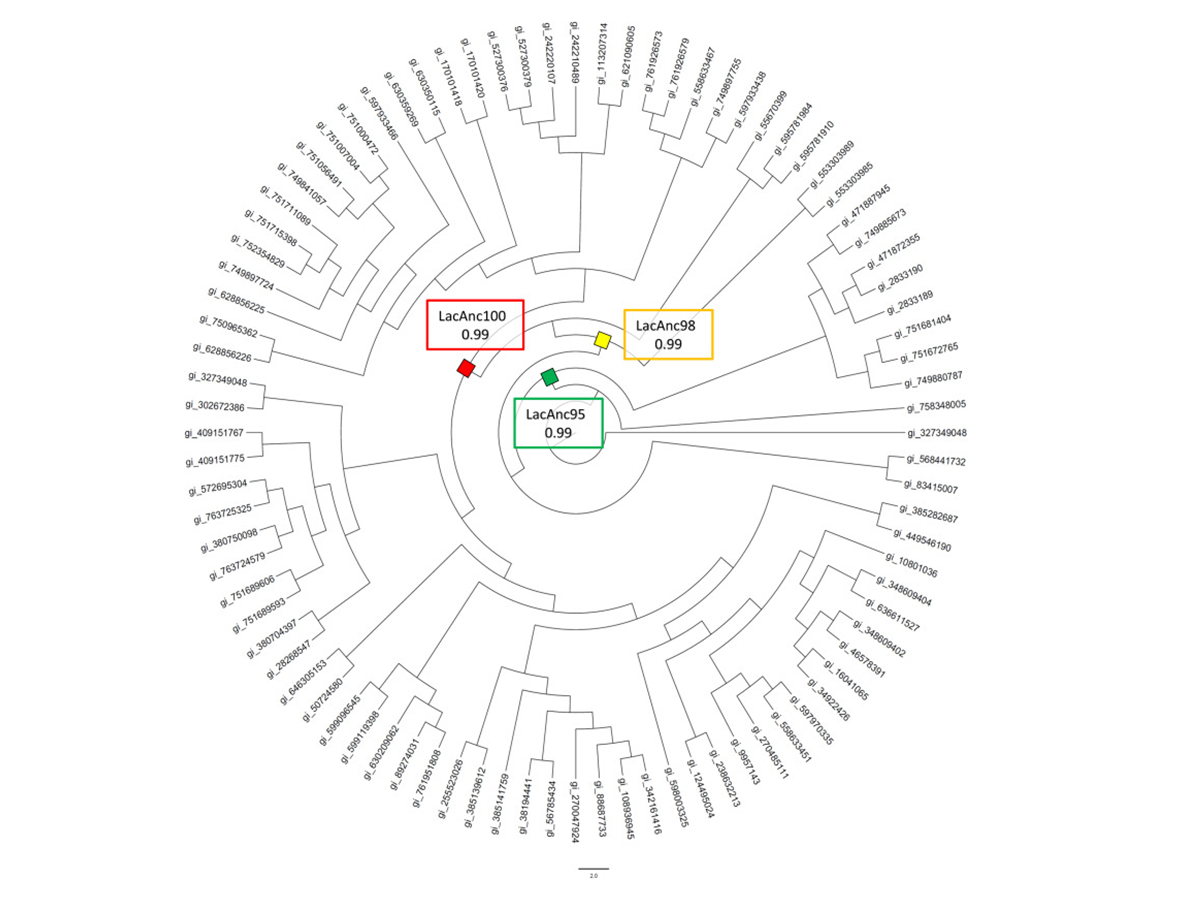
Ancestral Resurrection and Directed Evolution of Fungal Mesozoic Laccases
Gomez-Fernandez, B. J., Risso, V. A., Rueda, A., Sanchez-Ruiz, J. M., & Alcalde, M. (2020). Ancestral Resurrection and Directed Evolution of Fungal Mesozoic Laccases.
Applied and environmental microbiology, 86(14), e00778-20.
https://doi.org/10.1128/AEM.00778-20.
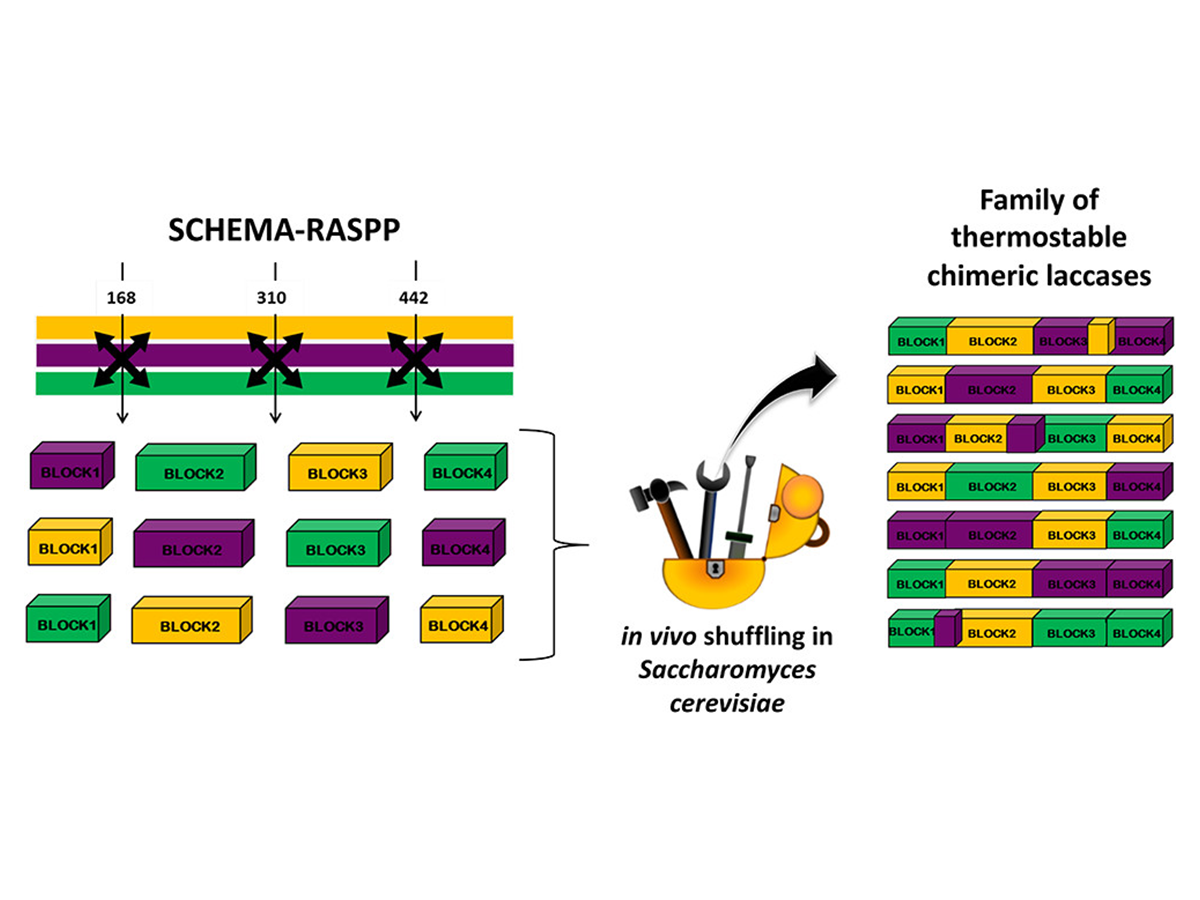
The Generation of Thermostable Fungal Laccase Chimeras by SCHEMA-RASPP Structure-Guided Recombination in Vivo
Mateljak, I., Rice, A., Yang, K., Tron, T., & Alcalde, M. (2019). The Generation of Thermostable Fungal Laccase Chimeras by SCHEMA-RASPP Structure-Guided Recombination in Vivo.
ACS Synthetic Biology, 8(4), 833-843.
https://doi.org/10.1021/acssynbio.8b00509.
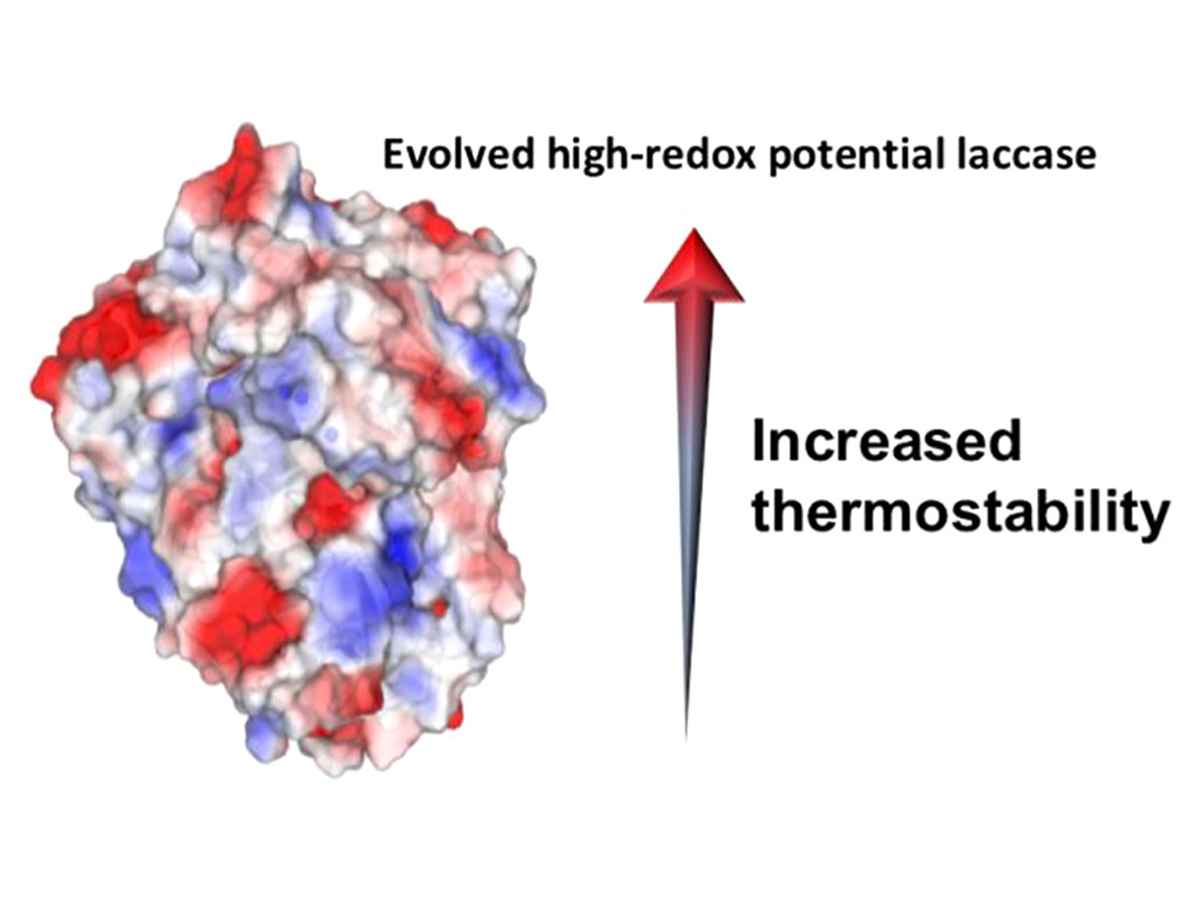
Engineering a Highly Thermostable High-Redox Potential Laccase
Mateljak, I., & Alcalde, M. (2021). Engineering a Highly Thermostable High-Redox Potential Laccase.
ACS Sustainable Chemistry & Engineering, 9(29), 9632-9637.
https://doi.org/10.1021/acssuschemeng.1c00622.